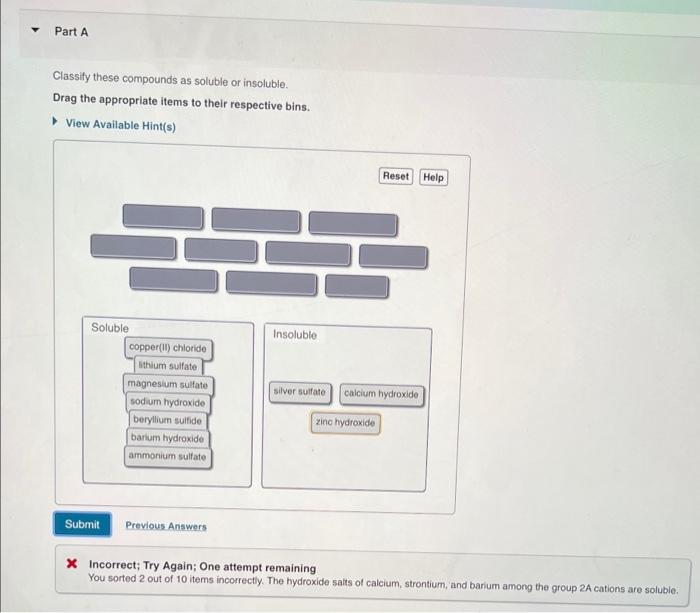
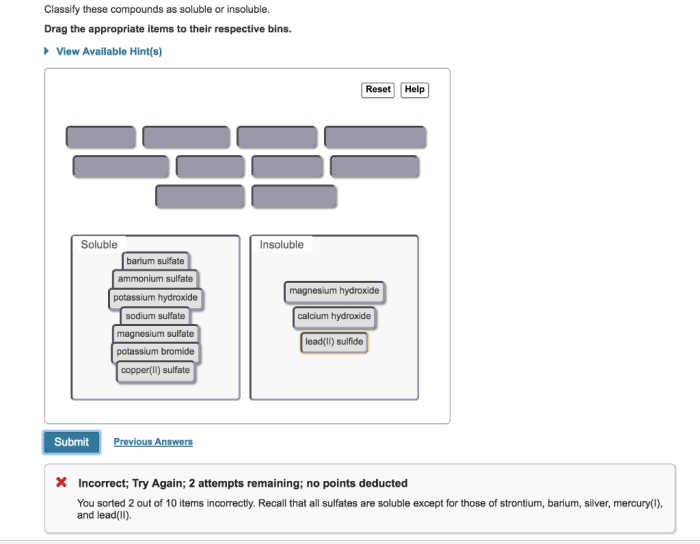
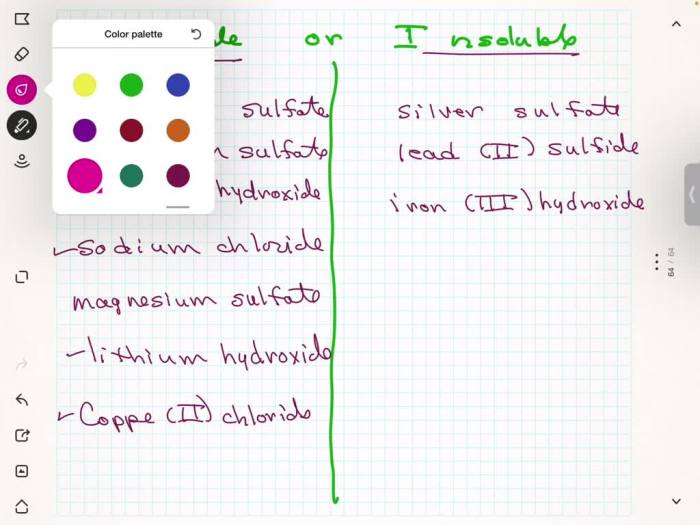
Classify these compounds as soluble or insoluble, delving into the intricacies of solubility and its dependence on various factors. Explore the role of molecular structure, polarity, and intermolecular forces in determining the solubility of organic and inorganic compounds.
Uncover the factors that influence solubility in water, including temperature, pH, and the presence of other solutes. Discover practical applications of solubility in diverse fields, ranging from drug design to environmental chemistry and industrial processes.
Solubility Concepts: Classify These Compounds As Soluble Or Insoluble
Solubility is the ability of a substance to dissolve in a solvent to form a homogeneous mixture. It depends on various factors, including molecular structure, polarity, and intermolecular forces.
Molecular structure influences solubility by affecting the surface area and polarity of the molecule. Polar molecules have a net charge or dipole moment, making them more soluble in polar solvents. Intermolecular forces, such as hydrogen bonding and van der Waals forces, can also affect solubility.
Classification of Compounds
Organic compounds are generally less soluble in water than inorganic compounds. Inorganic compounds often form ions, which can interact with water molecules through ion-dipole interactions.
- Soluble Organic Compounds:Alcohols, aldehydes, ketones, carboxylic acids, and amines
- Insoluble Organic Compounds:Hydrocarbons, ethers, esters, and lipids
- Soluble Inorganic Compounds:Alkali metal salts, halide salts, and nitrates
- Insoluble Inorganic Compounds:Carbonates, phosphates, and sulfates
Solubility in Water
Temperature generally increases solubility, as it increases the kinetic energy of molecules and disrupts intermolecular interactions. pH can also affect solubility, as it can change the charge of the solute and its interactions with water molecules.
The presence of other solutes can influence solubility through the common ion effect. If a solute has a common ion with the solvent, its solubility will decrease.
Examples of Soluble and Insoluble Compounds, Classify these compounds as soluble or insoluble
| Compound | Chemical Formula | Solubility in Water | Reason for Solubility/Insolubility |
|---|---|---|---|
| Sodium chloride | NaCl | Soluble | Ionic compound that forms ions in water |
| Glucose | C6H12O6 | Soluble | Polar molecule with multiple hydroxyl groups |
| Hexane | C6H14 | Insoluble | Nonpolar hydrocarbon with weak intermolecular forces |
| Calcium carbonate | CaCO3 | Insoluble | Ionic compound with strong lattice energy |
Applications of Solubility
Solubility has practical applications in various fields:
- Drug design:Optimizing solubility to enhance drug absorption and bioavailability
- Environmental chemistry:Understanding the fate and transport of pollutants in water
- Industrial processes:Controlling solubility for crystallization, extraction, and purification
Q&A
What factors affect the solubility of a compound?
Molecular structure, polarity, intermolecular forces, temperature, pH, and the presence of other solutes.
Why is solubility important in drug design?
Solubility determines the bioavailability and effectiveness of drugs in the body.