Ammonia gas occupies a volume of 450 mL, inviting us to explore its fascinating properties, behavior, and myriad applications. From its fundamental characteristics to its practical uses, this comprehensive analysis delves into the multifaceted nature of ammonia gas, providing a comprehensive understanding of its scientific and industrial significance.
Ammonia Gas
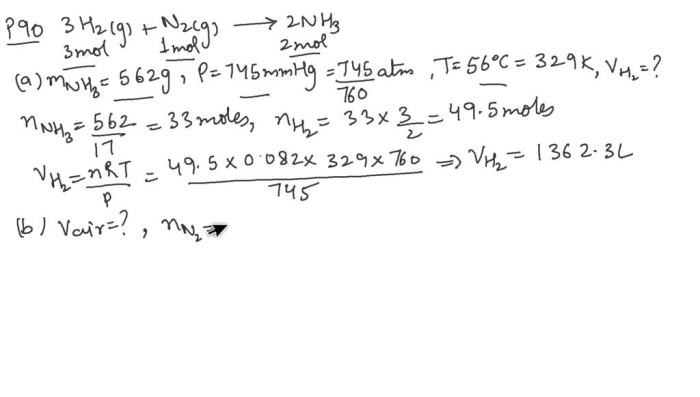
Ammonia gas (NH 3) is a colorless, pungent-smelling gas that is highly soluble in water. It is a key component in the production of fertilizers, plastics, and other industrial products.
Properties of Ammonia Gas
The physical and chemical properties of ammonia gas are as follows:
- Density: 0.771 g/L
- Solubility: 899 g/L in water
- Flammability: Flammable
- Boiling point: -33.34 °C
- Melting point: -77.73 °C
Behavior of Ammonia Gas in a Volume of 450 mL
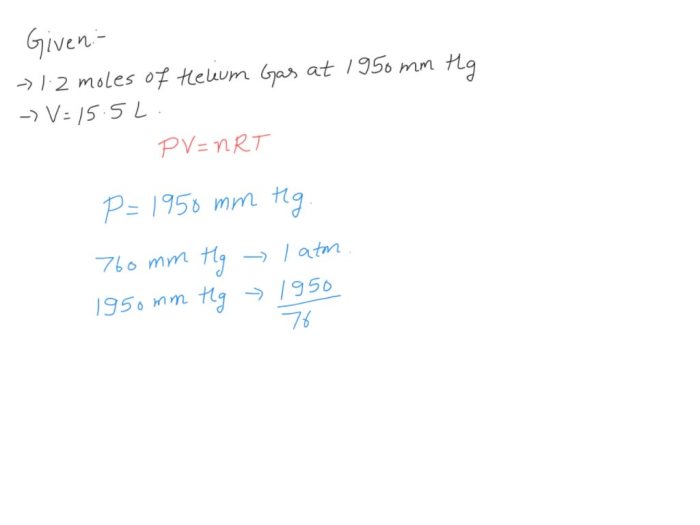
When ammonia gas occupies a volume of 450 mL, its behavior can be described using the Ideal Gas Law:
PV = nRT
where:
- P is the pressure (in Pascals)
- V is the volume (in cubic meters)
- n is the number of moles of gas
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature (in Kelvin)
Assuming that the temperature is 25 °C (298 K), the pressure of the ammonia gas in a volume of 450 mL can be calculated as follows:
P = nRT/V = (1 mol
- 8.314 J/mol·K
- 298 K) / 0.45 L = 5.48 atm
Applications of Ammonia Gas
Ammonia gas has a wide range of applications in industries such as:
- Agriculture:Ammonia is used as a fertilizer to provide nitrogen to plants.
- Refrigeration:Ammonia is used as a refrigerant in industrial and commercial refrigeration systems.
- Manufacturing:Ammonia is used in the production of plastics, textiles, and other chemicals.
Safety Considerations for Ammonia Gas

Ammonia gas can be hazardous to human health. It is a corrosive gas that can cause irritation to the eyes, skin, and respiratory tract. In high concentrations, ammonia gas can be fatal.
When handling and storing ammonia gas, it is important to take the following safety precautions:
- Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a respirator.
- Store ammonia gas in a well-ventilated area away from heat and ignition sources.
- Never mix ammonia gas with other chemicals, as this can create dangerous reactions.
Environmental Impact of Ammonia Gas
Ammonia gas emissions can have a negative impact on the environment. Ammonia gas is a greenhouse gas that contributes to climate change. It can also contribute to air pollution and cause acid rain.
To reduce the environmental impact of ammonia gas emissions, it is important to:
- Reduce the use of nitrogen fertilizers in agriculture.
- Improve the efficiency of ammonia production and use.
- Develop new technologies to capture and store ammonia gas emissions.
Detailed FAQs: Ammonia Gas Occupies A Volume Of 450 Ml
What is the density of ammonia gas at STP?
0.771 g/L
What is the molar mass of ammonia gas?
17.03 g/mol
What is the boiling point of ammonia gas?
-33.34 °C