Density of pennies lab answer key – In this lab, we will explore the density of pennies, a common object found in our everyday lives. By understanding the concept of density and how it applies to pennies, we can gain valuable insights into the physical properties of matter and their practical applications.
Density, a fundamental property of matter, is defined as the mass per unit volume. It provides a measure of how tightly packed the particles of a substance are. In this experiment, we will determine the density of pennies using a simple and engaging lab procedure.
1. Density of Pennies
Density is a measure of how tightly packed matter is within a given space. In the context of pennies, density refers to the amount of mass per unit volume of the coin. Understanding density is crucial for various applications, including engineering, material science, and everyday life.
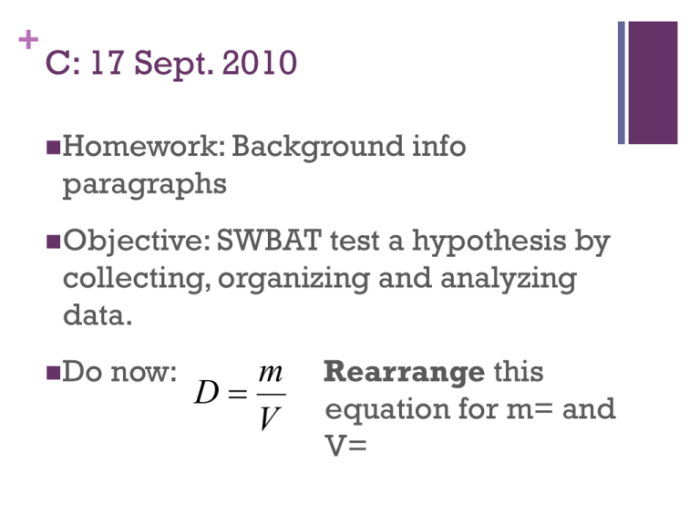
The formula for calculating density is:
ρ = m/V
where:
- ρ (rho) represents density, measured in grams per cubic centimeter (g/cm³)
- m represents mass, measured in grams (g)
- V represents volume, measured in cubic centimeters (cm³)
Density provides insights into the compactness of objects. For instance, a material with a higher density has more mass packed into a smaller volume compared to a material with a lower density.
2. Lab Experiment: Density Of Pennies Lab Answer Key
Materials:, Density of pennies lab answer key
- Pennies
- Graduated cylinder (100 mL or larger)
- Water
- Balance
- Calculator
Safety Precautions:
- Handle the pennies with care to avoid cuts.
- Wear gloves when handling the balance.
- Dispose of the pennies and water properly.
Procedure:
- Measure the mass of 10 pennies using the balance.
- Fill the graduated cylinder with approximately 50 mL of water and record the initial volume.
- Submerge the pennies in the water and record the new volume.
- Calculate the volume of the pennies by subtracting the initial volume from the new volume.
- Calculate the density of the pennies using the formula: ρ = m/V.
- Repeat steps 1-5 for multiple sets of 10 pennies to obtain an average density.
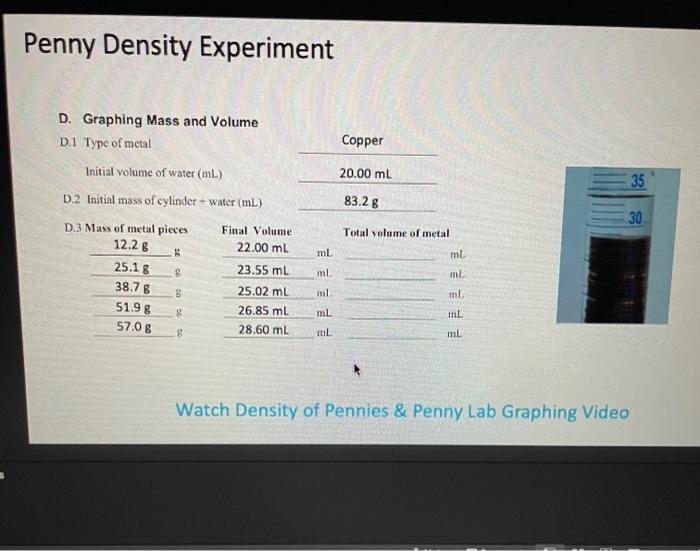
3. Data Analysis
Record the experimental data in the following table:
| Trial | Mass (g) | Volume (cm³) | Density (g/cm³) |
|---|---|---|---|
| 1 | |||
| 2 | |||
| 3 | |||
| … |
To calculate the average density, add up the density values for each trial and divide by the total number of trials.
Error can affect the accuracy of the results due to factors such as measurement uncertainties and variations in the pennies’ composition. To minimize error, ensure precise measurements and repeat the experiment multiple times.
4. Discussion

The density of pennies can vary slightly due to factors such as the composition of the alloy used and the manufacturing process. The composition of a penny is primarily copper, with small amounts of zinc and other metals.
Density is closely related to other physical properties, such as mass and volume. Objects with a higher density have a greater mass for their size compared to objects with a lower density. This relationship is important in various applications, such as buoyancy and fluid dynamics.
Density finds applications in fields like engineering and material science. For example, engineers consider density when designing structures and vehicles to ensure stability and performance. In material science, density is used to identify and characterize different materials.
Common Queries
What is the formula for calculating density?
Density = Mass / Volume
What are the units of density?
The SI unit of density is kilograms per cubic meter (kg/m³).
How does density affect the behavior of objects in water?
Objects with higher density than water will sink, while objects with lower density will float.
